Article Sharing|Recovery of Precious and Rare Metals from Anode Sludges in Non-Ferrous Metal Electrolytic Refining: Turning Sludge into Treasure
During the electrolytic refining process, precious metals and rare metals in anode plates do not dissolve in the electrolyte but instead precipitate as anode sludge. Although these black-gray sludges appear to be waste, their value far exceeds expectations, making them an important source of precious metals in the non-ferrous metallurgy industry.

1.The Mystery of Anode Sludge: Composition and Value
Anode sludge is the insoluble residue generated during electrolytic refining, typically accounting for 2% to 5% of the anode plate’s weight. Despite its small volume, it is a “rich bed” of precious metals.
The composition of anode sludge varies significantly depending on the source. Copper anode sludge generally contains 0.5%-1% gold, 5%-20% silver, 10%-25% copper, 2%-10% selenium, as well as lead, tin, arsenic and other elements.
Lead anode sludge is rich in gold, silver, bismuth, and other elements. The value of these precious metals makes anode sludge processing a significant profit source for non-ferrous metal smelting enterprises.
Processing methods for anode sludge mainly fall into three categories: traditional pyrometallurgical processes, hydrometallurgical processes, and combined beneficiation approaches. The choice of process depends on sludge composition, precious metal content, processing scale, and environmental requirements.
Table 1: Comparison of Three Processing Routes
| Process Route | Core Principle | Main Features | Applicability |
| Traditional Pyrometallurgy | High-temperature smelting concentrates precious metals into lead or copper, followed by further refining | Large processing capacity, mature technology, relatively complete recovery; but high energy consumption and environmental pressure | Suitable for large-scale processing, especially complex sludge |
| Hydrometallurgy | Selective dissolution and separation of precious metals using chemical reagents | Lower energy consumption, high direct metal recovery, environmentally friendly; but longer process and high reagent consumption | High environmental standards, medium-small scale, or as pre-treatment before pyrometallurgy |
| Combined Beneficiation | Physical pre-enrichment (e.g., flotation) followed by hydrometallurgical or pyrometallurgical treatment | Significantly reduces feed material to furnace, lowers subsequent processing costs and energy consumption | Pre-treatment to improve efficiency and economics of subsequent processes |
2.Pre-Treatment: Removal of Base Metals
The first step in anode sludge treatment is usually pre-treatment to remove base metals and enrich precious metals. The effectiveness of pre-treatment directly affects the efficiency and purity of subsequent precious metal recovery.
Oxidative roasting is a commonly used pre-treatment method. Roasting at high temperature volatilizes selenium, tellurium, and separates them, converting metals to easily processed oxides. This method is adaptable, uses simple equipment, has favorable operating conditions, and minimal environmental impact.
Sulfuric acid roasting is widely applied for selenium recovery and offers high selenium recovery rates, suitable for processing various feedstocks. During roasting, selenides convert to soluble selenates, facilitating subsequent aqueous leaching recovery.
Concentrated sulfuric acid leaching is an effective means to separate base metals. Under temperatures below 200°C, materials are treated with concentrated sulfuric acid, converting base metals to soluble sulfates, which are then diluted with water for leaching separation, while precious metals remain enriched in insoluble residues.
Controlling the leaching temperature is critical. Studies indicate that below 190°C, precious metals largely remain in insoluble residues, with losses controlled under 0.2%.
Oxidative leaching of copper and selenium is also common at this stage. Industrial experiments using sodium chlorate in dilute sulfuric acid oxidatively leach approximately 92% copper and 86% selenium from the sludge.
3.Precious Metal Enrichment: Pyrometallurgical and Hydrometallurgical Routes
Following pre-treatment, precious metals in the sludge are preliminarily enriched and require further extraction, mainly via pyrometallurgical or hydrometallurgical processes.
3.1 Pyrometallurgical Process
Traditional pyrometallurgical treatment includes reductive smelting, oxidative refining, and electrolytic refining.
Reductive smelting is performed at 1200–1300°C with reducers and fluxes added, concentrating precious metals into lead or copper alloys known as “precious lead.”
Precious lead is then oxidatively refined in a silver refinery furnace to remove residual copper, tellurium, arsenic, and other impurities, producing a gold-silver alloy plate.
The gold-silver alloy undergoes electrolytic refining: silver is refined electrolytically to pure silver, while gold concentrates in anode sludge for further processing to obtain pure gold.
Pyrometallurgical processes handle large volumes, are technologically mature, and have high recovery rates but suffer from high energy consumption and environmental pressure.
3.2 Hydrometallurgical Process
Hydrometallurgy selectively dissolves and separates precious metals using chemical reagents, avoiding high-temperature smelting, and has gained increasing attention recently.
Chlorination is central to hydrometallurgy. One approach uses sodium chlorate oxidation in hydrochloric acid solution: gold is chlorinated into solution, silver remains in residue, enabling selective gold extraction.
Gold chloride solutions are enriched and purified by solvent extraction (e.g., with methyl isobutyl ketone), then reduced by agents such as oxalic acid or sulfur dioxide to obtain gold powder.
The residue after gold extraction can be treated with ammonia leaching to extract silver, which forms silver-ammonia complexes in solution; subsequent reduction by hydrazine hydrate yields sponge silver.
This sodium chlorate gold leaching-ammonia silver leaching hydrometallurgical process applied to copper anode sludge achieves gold and silver leaching rates exceeding 98%.
4.Recovery of Rare Metals: Selenium and Tellurium Extraction Technologies
Recovery of rare metals such as selenium and tellurium from anode sludge has significant economic value. Sulfuric acid roasting is the mainstream industrial technology for selenium recovery.
During roasting, selenium oxidizes to volatile selenium dioxide, collected in absorption devices, then reduced with sulfur dioxide to elemental selenium (oxidation + reduction).
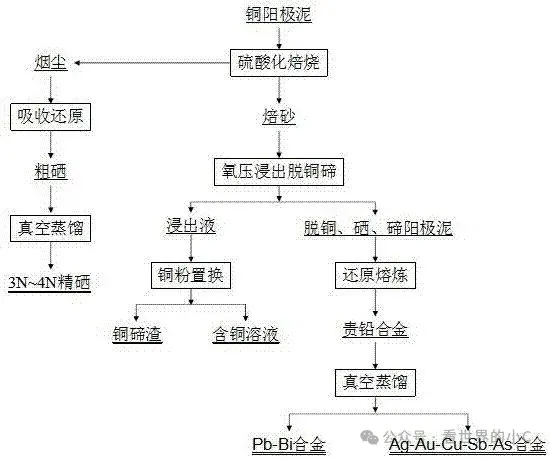
(A sulfuric acid roasting method and process for recovering selenium, tellurium, and precious metals from copper anode sludge.)
For selenium recovery from solution, the selenium-containing solution is adjusted to appropriate acidity and iron filings are added to reduce and precipitate most selenium as elemental selenium. About 80% of selenium in solution can be reduced to selenium precipitate; residual selenium can be further reduced by adding a small amount of sodium chlorate.
Tellurium recovery usually follows selenium recovery, with corresponding enrichment and reduction steps depending on its process behavior.
A method for pre-treatment and recovery of dispersed metals from anode sludge demonstrates that through reasonable process design, acid leachate can be reduced to recover selenium and tellurium, or after direct alkali reaction in leachate, selenium-tellurium residues can be obtained.
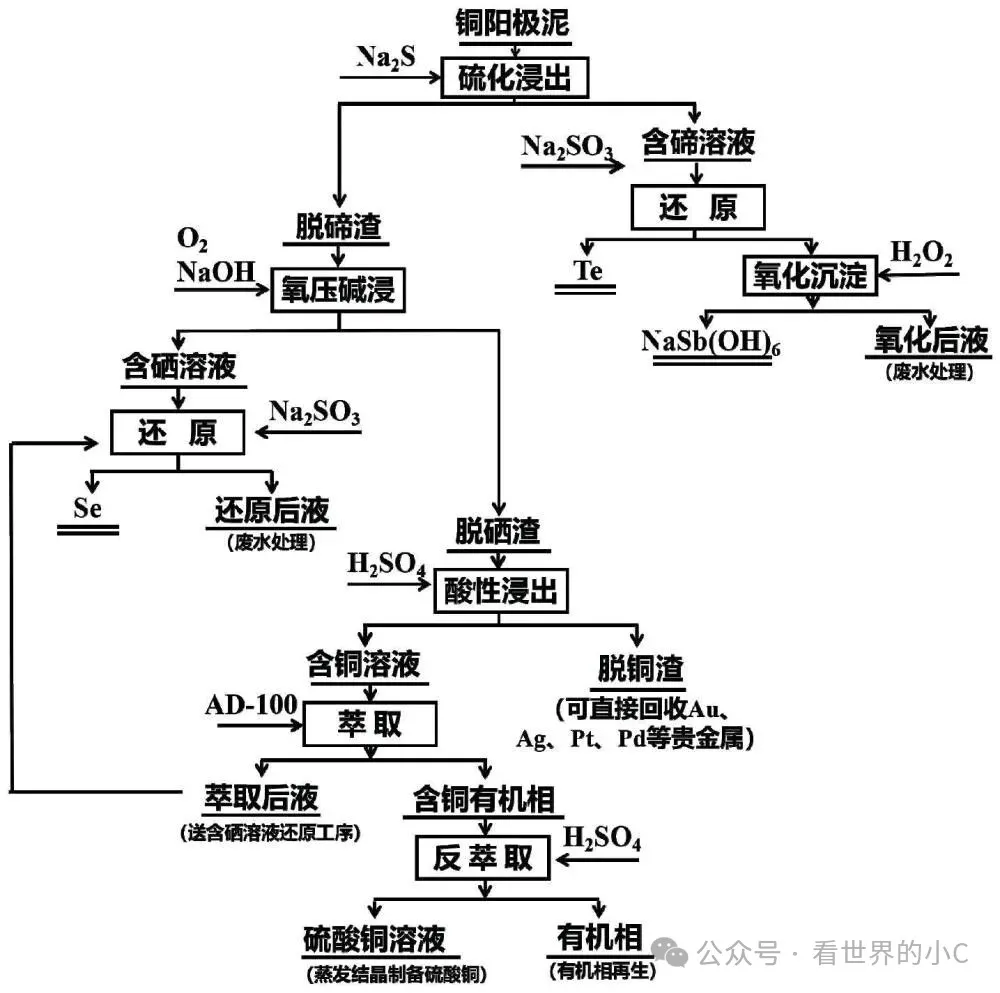
(A fully hydrometallurgical stepwise efficient method for separating and recovering copper, selenium, and tellurium from copper anode sludge.)
Dispersed metals such as selenium, tellurium, and bismuth have extremely important uses and are key components in today’s high-tech new materials.
5.Process Comparison and Development Trends
Pyrometallurgical processes have strong adaptability and high metal recovery rates but suffer from high energy consumption and environmental pressure; hydrometallurgical processes feature high direct metal recovery and relative environmental friendliness but have longer process times and high reagent consumption.
Development trends indicate that short-process, high-efficiency methods are desirable. For example, a “one-step chlorination” full hydrometallurgical process uses sodium chlorate chlorination of anode sludge in hydrochloric acid, dissolving gold for solvent extraction and stripping to obtain gold powder; chlorinated residue is ammonia-leached to dissolve silver, then reduced to sponge silver.
Green environmental protection and comprehensive resource utilization are increasingly emphasized. Modern anode sludge treatment technologies focus on three-waste management and full-element recovery. For example, electrolytic purification of leachates is more economical and capacity-enhancing than traditional cyclone electrolytic precipitation systems, while reducing environmental risks.
Technology integration and optimization continue to advance. Combining pyrometallurgical and hydrometallurgical methods leverages strengths and avoids weaknesses, saving energy and reducing consumption, improving safety, environmental performance, and precious metal recovery rates.
Efficient enrichment of precious metals from complex low-grade chlorination residues can increase concentration factors and recovery rates of gold, platinum, palladium, and other precious metals, achieving enriched residues with precious metal grades of 9000–15000 g/t and recovery rates above 98%.
With technological progress and stricter environmental requirements, anode sludge treatment is developing towards higher efficiency, cleaner processes, and greater economy. Increasingly, enterprises adopt hydrometallurgical methods to reduce environmental issues from pyrometallurgy while optimizing processes to improve comprehensive resource recovery.
Facing increasingly scarce global precious metal resources, this once dark and seemingly worthless anode sludge has become a true “urban mineral.”
